7.4. The Carbonate Pump#
Key Points:
Many plankton species make shells or skeletons from calcium carbonate (CaCO3).
The process of calcification takes up CO32- from the surface ocean and exports it to the deep.
Export of CO32- reduces TA and DIC in a 2:1 ratio in the surface, and increases them in the deep, in a process known as the carbonate pump.
Calcification lowers ocean pH, shifting carbon speciation and causing the release of CO2 to the atmosphere.
Calcification#
Many groups of marine organisms make shells or skeletons from calcium carbonate (CaCO3). You are probably familiar with bivavles and gastropods that produce the shells you find on the sea shore, and corals that produce massive reef structures found in tropical coastal environments. Some examples you may not have encountered are the planktonic organisms found throughout the surface ocean that produce microscopic CaCO3 structures. Three groups of marine plankton are responsible the majority of calcification in the open ocean (Fig. 7.25):
Coccolithophores are small (3-50 μm) phytoplankton that produce intricate calcium carbonate plates called coccoliths. Coccolithophores are a major calcifyer in the open ocean, demonstrated by the fact that coccoliths are the main constituent of massive chalk deposits found in uplifted marine sediments (e.g. the White Cliffs of Dover). The exact purpose of these coccoliths is unclear, although hypotheses include buoyancy control, protection from grazing and light scattering.
Foraminifera are slightly larger (50-500 μm) unicellular zooplankton which produce calcium carbonate shells called tests. These tests have a distinctive multi-chambered structure, which grows gradually throughout the organism’s life cycle. Some species, like the one pictured below, also create long mineralised spines that are home to symbiotic algae.
Pteropods are larger-still (0.5-10 mm) multicellular zooplankton who’s name literally translates to ‘wing foot’. Pteropods are gastropods, which (mostly) produce a recognisable calcium carbonate ‘snail shell’ structure, with the soft parts of the body adapted into ‘wing’ that allow them some limited movement through the water.

Fig. 7.25 The three main open-ocean calcifiers. An SEM micrograph of the coccolithophore Emiliania huleyi, and light micrographs of the planktic foraminifera Orbulina universa and a Pteropod. Images from wikimedia commons (coccolithophore, pteropod) and Oscar Branson.#
The shells of these organisms are formed from one of two ‘polymorphs’ of CaCO3 that are stable at Earth surface conditions: calcite and aragonite. Coccolithophores and foraminifera produce calcite, while pteropods produce aragonite.
The formation of these shells is actively controlled by the organism, and involves taking up dissolved Ca2+ and CO32- ions from the surrounding water, and using them to build the a mineral structure. This process is known as calcification, and can be written as:
Calcification and CO2#
Removing CO32- from the water and trapping it in mineral form both directly removes DIC from surface water, and alters the speciation of the remaining DIC in the surface ocean, causing CO2 to be released to the atmosphere, further reducing surface DIC.
Just like organic matter in The Biological Pump, the CO32- trapped in shells is exported from the surface ocean as shell-bearing organisms die and sink. The uptake and export of CO32- directly removes DIC from the surface ocean.
We also saw when considering The Solubility Pump that the DIC species exist in an equilibrium with each other and pH. Removing CO32- causes this speciation adjust, reducing pH because a HCO3- dissociates to replace it, releasing H+ into the solution. We can re-draw DIC speciation diagram (Fig. 7.10) to see how the abundance of CO32- relates to the concentration of the other species (Fig. 7.26):
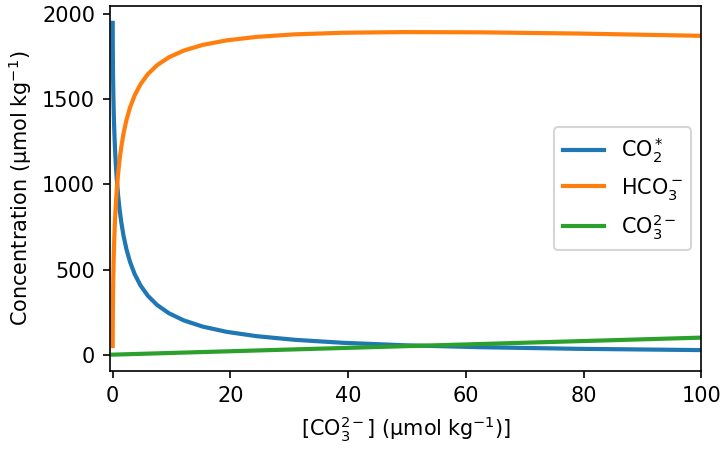
Fig. 7.26 Change in DIC speciation as a function of CO32- concentration. This equilbrium is calculated at constant DIC concentration - there is no gas exchange with the atmosphere. If gas exchange were allowed to happen CO2 would be released to the atmosphere as CO32- is removed from the surface ocean. This is shown in Fig. 7.27.#
Removing CO32- from the water causes the fraction of DIC that exists as CO2 to increase (the equilibrium shifts to the left in Fig. 7.26), causing CO2 to be released to the atmosphere. Calcification therefore removes more DIC that we would predict based solely on the uptake of CO32- from the surface ocean, because its effect on DIC speciation make it a source of CO2 to the atmosphere (Fig. 7.27).
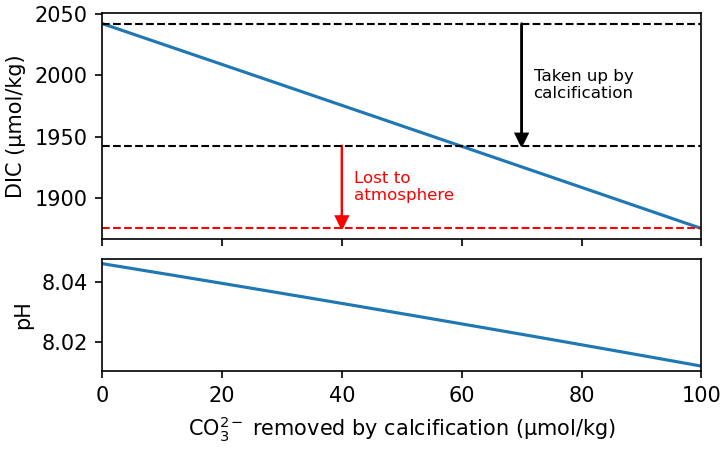
Fig. 7.27 The change in pH and DIC caused by the removal of CO32- from the surface ocean by calcification when it is equilibrating with an atmosphere at 400 ppm pCO2. We can see that DIC is reduced directly by the removal of CO32-, and indirectly by the resulting shift in DIC speciation (Fig. 7.26), causing CO2 to be released to the atmosphere.#
The mechanism of exporting of CO32- from the surface ocean via the formation and sinking of CaCO3 structures is intuitive, but how is CO32- released back into the deep ocean? The bacterial breakdown that applies to organic matter doesn’t apply here, because there is no energetic or nutritional benefit for the microbes to actively break down CaCO3. To consider the remineralisation of CaCO3, we must briefly consider mineral solubility and saturation states.
Saturation State#
Just like the DIC speciation equations that drive The Solubility Pump (7.9), the calcification reaction (7.24) has an equilibrium point that is defined by the thermodynamic properties of the reactants and product, and is described by an equilibrium solubility product (\(K_{sp}\)) for the mineral phase:
Which is the concentration of Ca2+ and CO32- at which the mineral is in equilibrium with the surrounding water, and will neither grow nor dissolve. Note that, just like \(K_1\) and \(K_2\), \(K_{sp}\) varies with temperature, salinity and pressure. From this, we can define the saturation state of seawater with respect to CaCO3:
Values of \(\Omega > 1\) will favour the formation of CaCO3, while values of \(\Omega < 1\) will favour dissolution.
Ca is relatively abundant and constant in seawater (~10.2 mmol kg-1) compared to CO32- (0.1-0.3 mmol kg-1 at normal ocean surface conditions). This means that the formation and dissolution of CaCO3 is predominantly controlled by the concentration of CO32- in the water.
The majority of the modern surface ocean is saturated (i.e. \(\Omega > 1\)) with respect to CaCO3, and so calcification is favoured. For CaCO3 to dissolve in the deep ocean, the deep ocean must be undersaturated (i.e. \(\Omega < 1\)) with respect to CaCO3.
CaCO3 Dissolution#
The dissolution of CaCO3 is driven by a combination of changes in pressure and the impact of the biological pump.
Pressure#
The CaCO3 minerals become more soluble at higher pressure; \(K_{sp}\) increases with depth. The two polymorphs of CaCO3, calcite and aragonite, also have different \(K_{sp}\) values - calcite is more stable than aragonite, so has a lower \(K_{sp}\). The speciation of DIC is also sensitive to pressure, which causes [CO32-] to decrease with depth. In combination, this means that as a CaCO3 particle sinks through the ocean it will become less stable as pressure increases, while [CO32-] in the surrounding water simultaneously decreases. This causes the mineral to dissolve when it sinks below the depth at which [CO32-] is lower than \(\frac{K_{sp}}{[Ca^{2+}]}\) (Fig. 7.28). Thus, changes in pressure alone are sufficient to dissolve a large fraction of the CaCO3 in the deep ocean.
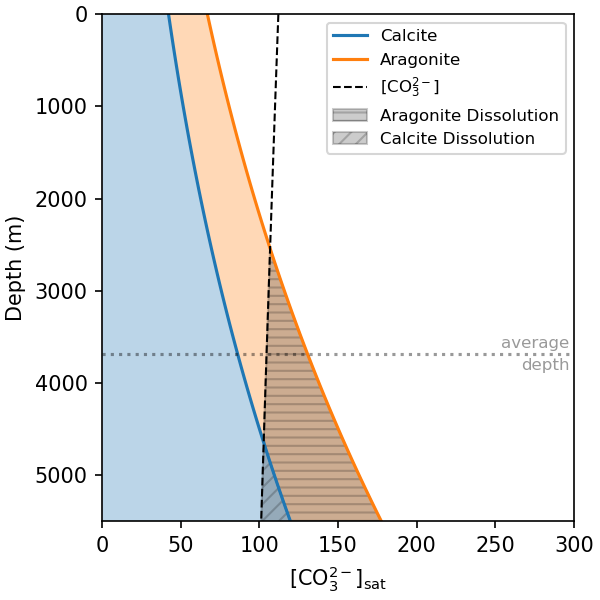
Fig. 7.28 The concentration of CO32- in seawater at which the CaCO3 minerals calcite and aragonite are in equilibrium with seawater across a range of water depths. The shaded regions are supersaturated with respect to each mineral. The horizontal dashed line shows the [CO32-] for typical deep ocean water. The equilibrium [CO32-] for each mineral both increase with depth, while [CO32-] decreases with depth. The mineral will begin to dissolve below where the dashed line intersects with the solid lines. Calculated at 4 °C and salinity of 35.#
Biological Pump#
The effects of pressure on solubility and speciation then combine with the biological pump to make CaCO3 dissolution occur at shallower depths than would be expected from pressure alone. The uptake of CO2 by photosynthesis in surface waters raises pH and [CO32-], and the release of CO2 by remineralisation in the deep ocean causes pH and [CO32-] to decrease. In combination with the pressure effects on CaCO3 solubility, this causes CaCO3 dissolution to occur at shallower depths (Fig. 7.29)}).
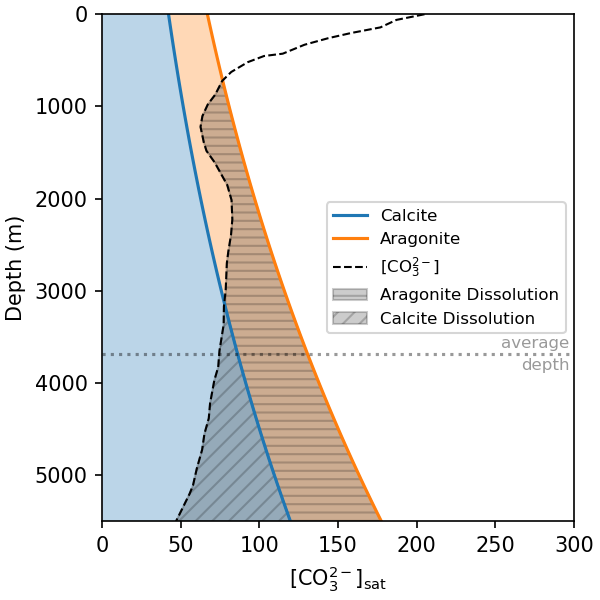
Fig. 7.29 Similar to Fig. 7.28, but showing a real [CO32-] profile for the Southern Atlantic. The action of the biological pump causes [CO32-] to be elevated in surface waters, where CO2 is being removed by photosynthesis, and elevated at depth, where CO2 is being released by remineralistion.#
Aggregates and Local Environments#
Another layer of complexity when considering CaCO3 is the effect of particle aggregates. Most material leaving the surface ocean is in the form of aggregates - collections of smaller particles that clump together to form larger particles. The respiration of bacteria in these aggregates can drive very high local CO2 concentrations, dramatically reducing pH and CO32- and creating a highly locally undersaturated environment. This effectively ‘concentrates’ the impact of the biological pump in a way that you would not pick up from bulk water chemistry measurements. A lot of CaCO3 dissolution occurs within these local environments, which can cause a substantial fraction of CaCO3 to dissolve much shallower than predicted by pressure and bulk water chemistry alone.
The Carbonate Pump#
The export of CaCO3 from the surface ocean to the deep ocean is known as the carbonate pump. The impact of the carbonate pump on carbon concentration and speciation considered above can also be described in terms of DIC and TA, which describe the concentration and speciation of carbon in the water. The carbonate pump acts to move TA and DIC from the surface to the deep ocean in a 2:1 ratio. This follows from our definitions of DIC and TA:
Because calcification and dissolution affect TA and DIC in different amounts, it will alter both the concentration and speciation of DIC in the water (Fig. 7.13).
The carbonate pump is inextricably connected to the biological pump, because CaCO3 production is driven by biological productivity. This means that the carbonate pump also depends on the supply of nutrients to the surface ocean, which fuels biological productivity. However, this connection is not one-way: the carbonate pump can also affect the efficiency of the biological pump by altering the density of sinking particles.
Ballasting#
We saw in The Biological Pump that the proportion of primary production that is exported to the deep ocean depends to a large extent on particle sinking speed, which is a function of particle density. Seawater has a density of ~1025 kg m-3, organic matter has a typical density of ~1030-1500 kg m-3. Calcite has a density of 2710 kg m-3. The inclusion of CaCO3 in particle aggregates will therefore act to increase their density, allowing them to sink faster and increasing the proportion of primary production that is exported to the deep ocean. This means that, even thought calcification is an immediate source of CO2 to the atmosphere, increasing the efficiency of the biological may offset this effect and make calcification a net (indirect) sink for CO2. This feedback is known as ballasting, and the amount of dense CaCO3 relative to organic matter in sinking particles is a critical parameter in determining the efficiency of the biological pump.
Ocean Acidification#
In The Solubility Pump we saw that pCO2 and pH are inversely related, and that elevating atmospheric pCO2 will cause the surface ocean to become more acidic. This would reduce [CO32-], and therefore the saturation state (Ω) of the surface ocean (Fig. 7.30). Reducing the saturation state has the potential to reduce CaCO3 production, by marine organisms, and enhance CaCO3 dissolution at shallow depths, thus reducing the strength of the carbonate pump.
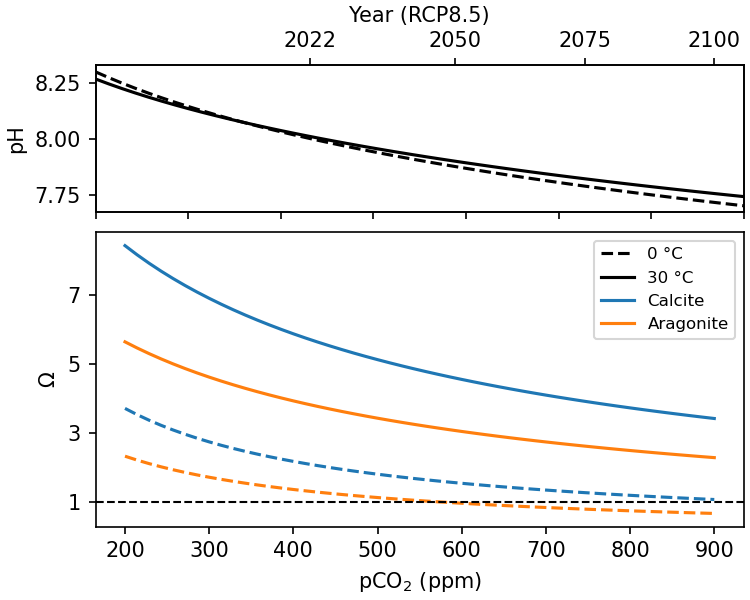
Fig. 7.30 The impact of ocean acidification on surface ocean \(\Omega\) at 0 and 30 °C. Because of the influence of temperature on solubility and DIC speciation, the high latitudes are much more affected by ocean acidification than the tropics. The polar waters may become undersaturated with respect to aragonite as soon as ~2050.#
In the immediate term, this could act as a negative feedback on atmospheric pCO2, because reductions in calcification will reduce the rate of associated CO2 release from the surface ocean. However, when the effect of CaCO3 production on ballasting the biological pump is considered, it is less clear whether impact of ocean acidification on the carbonate pump will have a net postitive or negative effect on atmospheric pCO2. The interaction between ballasting and ocean acidification is a significant source of uncertainty in our predictions of the uptake of CO2 by the ocean in future.
Modelling the Carbonate Pump#
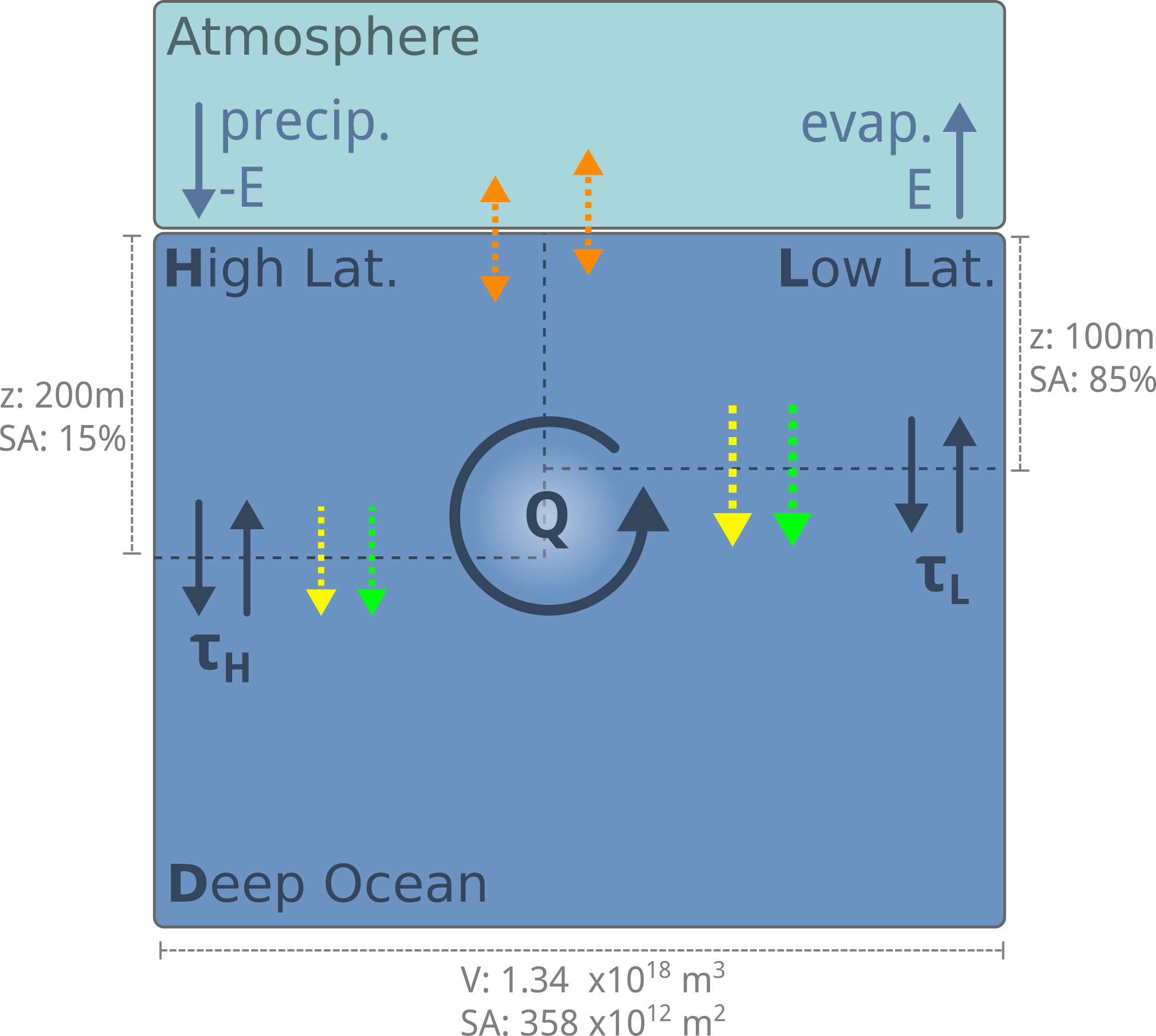
Fig. 7.31 To model the carbonate pump, we need to include the production of CaCO3 by biological life, which takes up DIC and TA in the surface ocean and exports it to the deep ocean.#
Calcification is biologically-mediated, and takes up CO3-2 from the surface ocean and exports it to the deep. We will therefore model calcification as a function of biological productivity in the surface ocean, assuming that some fraction of biological carbon export (\(f_{calc}\)) is accompanied by calcification:
The amount of CO32- exported from a surface box is therefore given by:
From our definitions of TA and DIC (7.25), this modifies TA and DIC in our model in a 2:1 ratio. We therefore model the impact of calcification on DIC as:
where the terms in square brackets represents the transport (7.2), gas exchange (7.16) and biological pump (7.22) terms. For TA, the expression is included as:
